Enabling Discoveries and Sharing Results
The Human Trisome Project creates a precision medicine platform that accelerates discoveries across numerous biomedical research fields. Our team and our collaborators constantly have new and exciting results to publish and share with the community.
Snapshots of some of our most recent results are below:
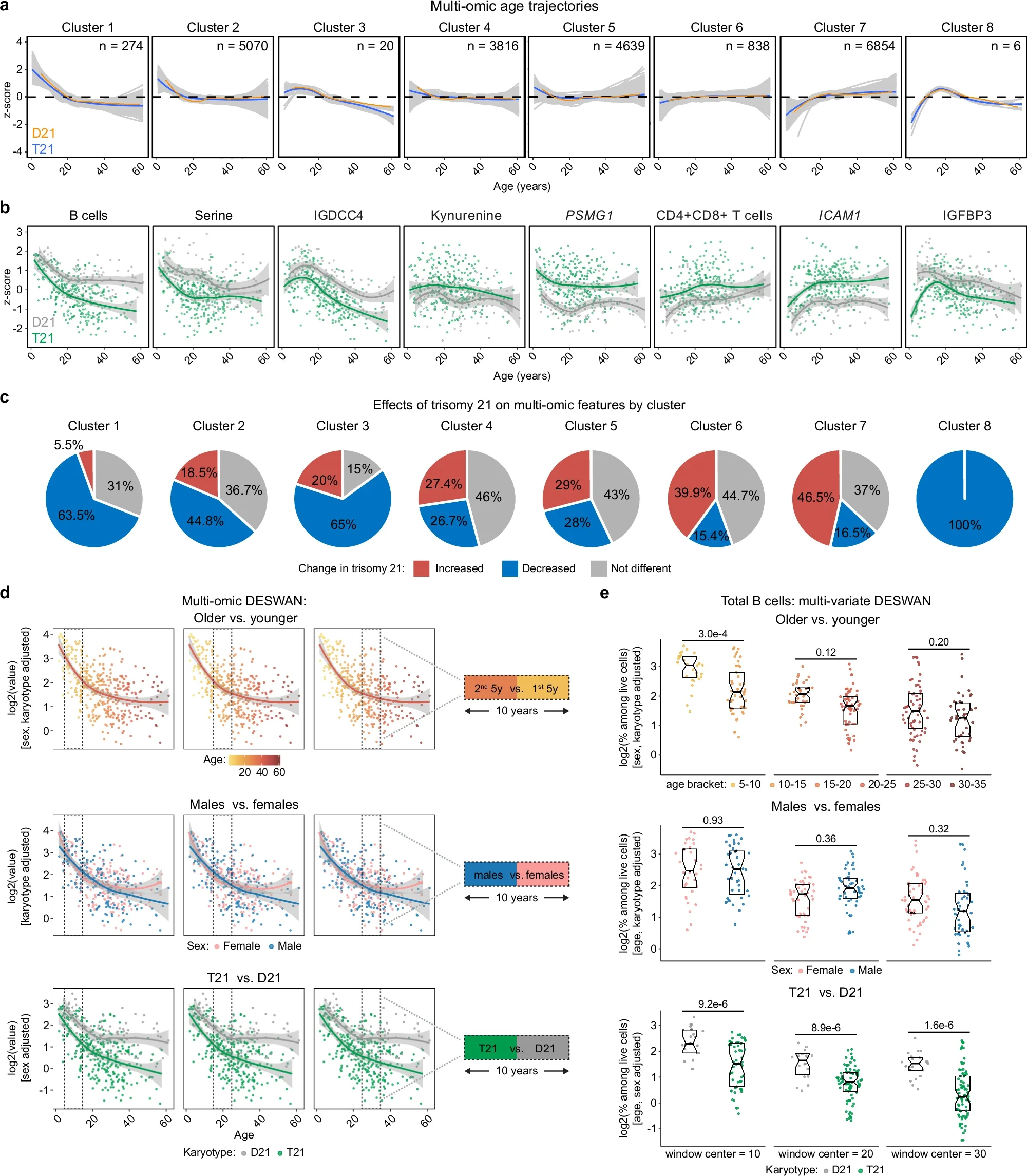
New groundbreaking research from the Crnic Institute reveals how trisomy 21 shapes biology across the lifespan.
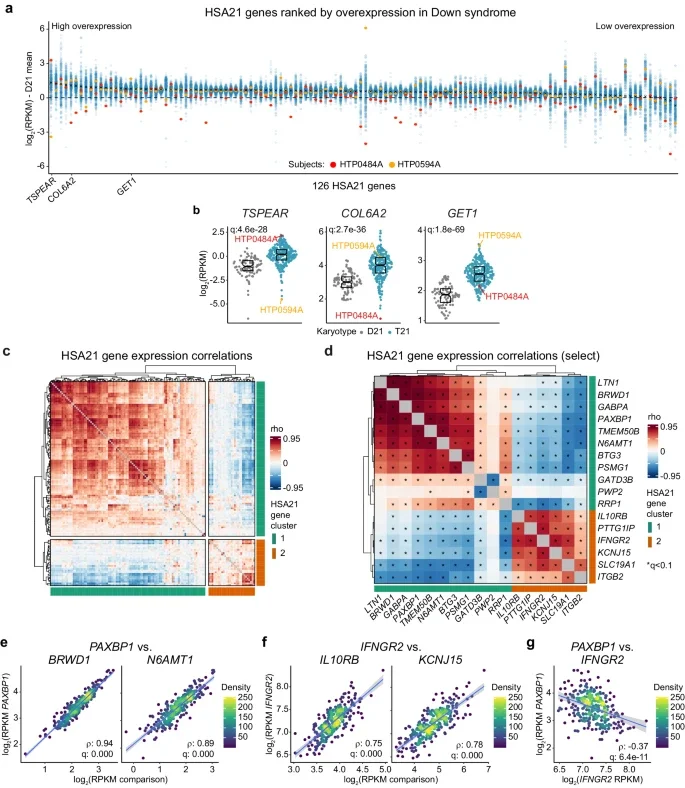
Crnic Institute researchers report an investigation of human chromosome 21 gene overexpression in hundreds of research participants with Down syndrome, which led to the identification of two major subsets of co-expressed genes.
Researchers provide evidence that a mitochondria-to-cilia signaling axis important during the earliest steps of neurogenesis is dysregulated in DS, yet remains amenable to small molecule intervention.
Congenital heart defects (CHDs) are the most common structural birth defect and are present in 40%–50% of children born with Down syndrome (DS).
People with DS experience co-occurring conditions at higher rates than the euploid population. However, the interplay between immune and metabolic alterations and the clinical manifestations of DS are poorly understood.
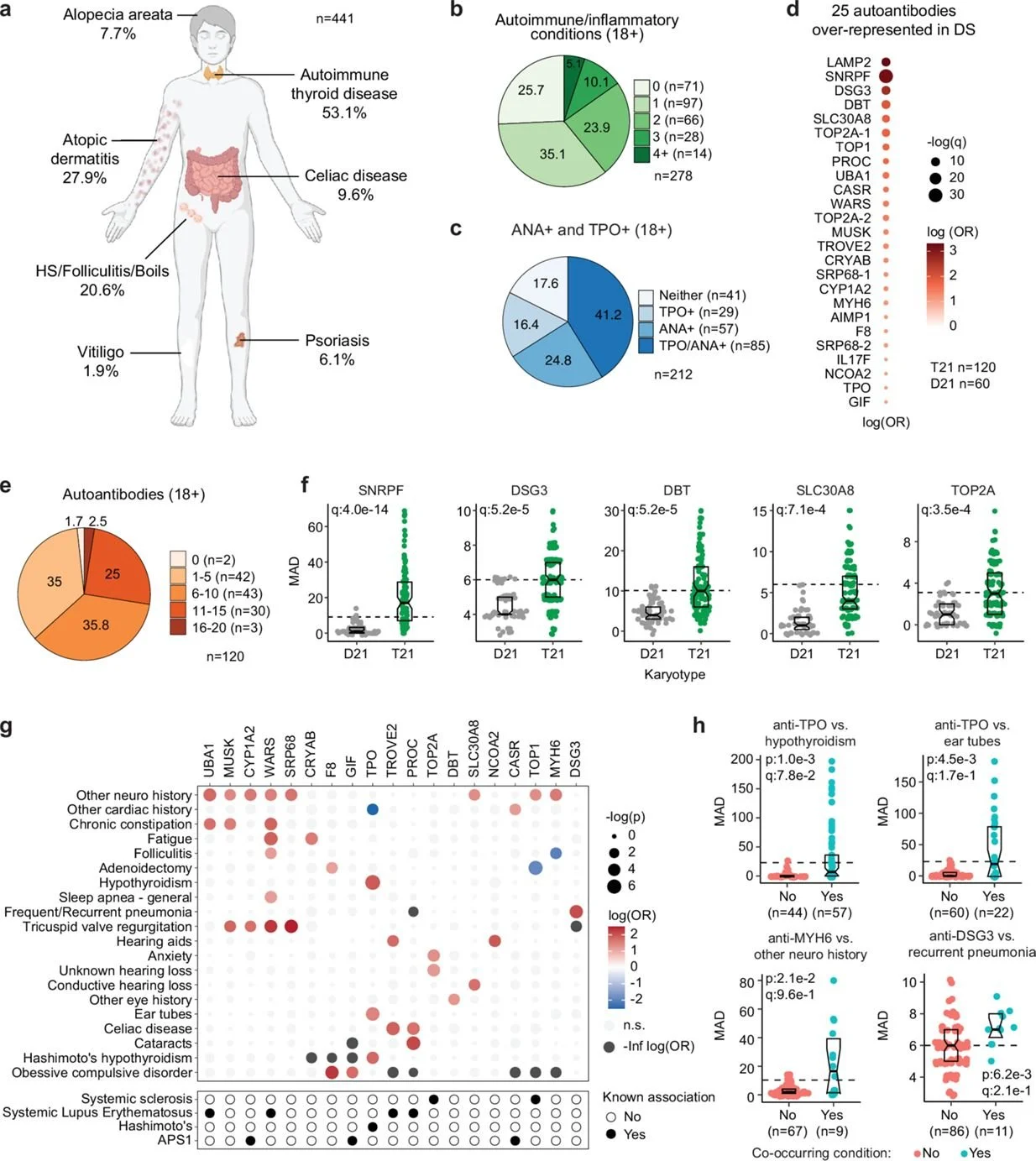
Crnic Institute study provides an important manuscript that includes two major advances in understanding immune dysregulation in a large cohort of individuals with Down syndrome.
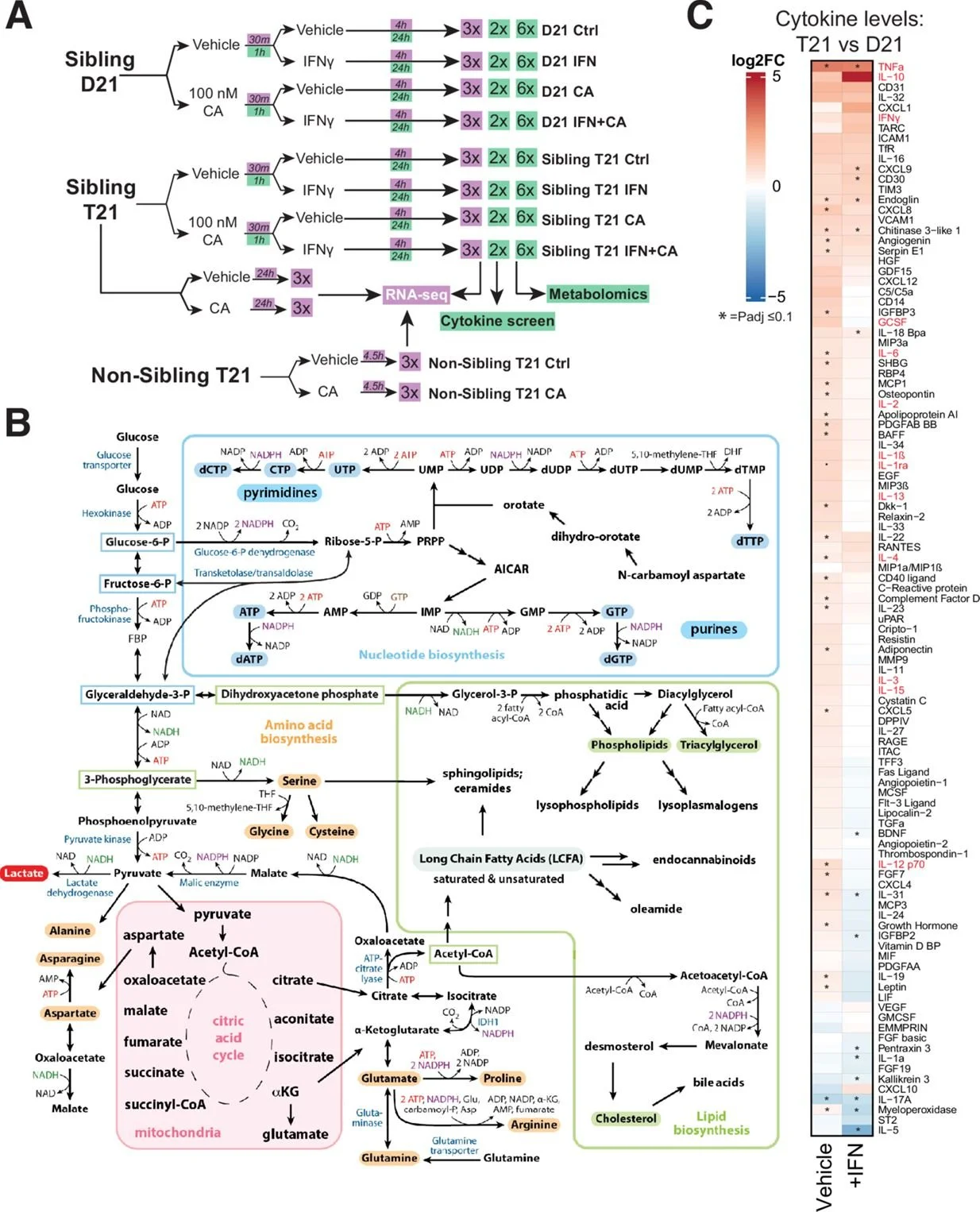
This is an important study providing compelling evidence that the Mediator kinase module mediates an elevated inflammatory response, manifested by heightened cytokine levels, associated with Downs syndrome (DS) via transcriptional changes impacting cell signaling and metabolism that involve mobilization of nuclear receptors by altered lipid metabolites, which has significance for the treatment of DS and other chronic inflammatory conditions.
New results reveal lifelong dysregulation of key oxygen-related processes that could contribute to the developmental and clinical hallmarks of DS.
New findings represent evidence for altered hepatic metabolism in DS that could be modulated by diet.
Crnic Institute study reveals immune dysregulation in Down Syndrome and potential therapeutic strategy.
The lack of easily measurable biomarkers remains a challenge in executing clinical trials for diabetic neuropathy (DN). Plasma Neurofilament light chain (NFL) concentration is a promising biomarker in immune-mediated neuropathies. Longitudinal studies evaluating NFL in DN have not been performed.
In the Dp16 mouse model, the Crnic Institute recently showed that normalization of copy number of the Ifnr cluster prevented heart malformations, but the mechanisms by which hyperactive IFN signaling may disrupt normal heart development await elucidation.